Role of microglia and strategies of neuroprotection in neonatal brain damage
Group Juliette Van Steenwinckel and Pierre Gressens

Lorem ipsum
Dolor sit amet, consectetuer adipiscing elit, sed diam nonummy nibh euismod tincidunt ut laoreet dolore magna aliquam erat volutpat. Ut wisi enim ad minim.
© Lorem ipsum dolor sit amet, consectetuer adipiscing
As brain-resident immune cells, microglia have the ability to sense the brain environment and adapt functions to maintain homeostasis. Microglia is also critically involved in neurodevelopmental processes required for proper brain maturation and function. Any perpetuation of these brain-building roles by early exposure to any environmental stress could explain long-term consequences on neurological abilities seen in neurodevelopmental diseases (ND) associated to prematurity or neonatal traumatic brain injuries (TBI).
In a mouse model of encephalopathy of prematurity (Eop) induced by early exposure to inflammation we recently demonstrated that microglial have regional-specific pattern of reactivity and drive hypomyelination, the main feature of Eop (Klein L. et al., Glia 2022 ; Van Steenwinckel J. et al., Brain 2019). We implemented a specific study within Eop’s mouse hippocampi that supported the onset of specific pro-inflammatory processes inducing a decrease in the proliferation of the Tbr2+ intermediate progenitors and later persistent hippocampal dependent behaviors including increased signs of anxiety and long-term spatial memory deficits (Veerasammy S. et al., Brain Behav Immun Health 2020).
Using large-scale analysis of microglia transcriptomic modifications, we identified original and promising molecular systems to modulate microglial reactivity including Dlg4 encoding the post-synaptic density 95 protein (Krishnan M.L. et al., Nat Commun 2017), the WNT pathway (Van Steenwinckel J. et al, Brain 2019) and the immunomodulatory microRNA 146b-5p (Bokobza C. et al., Ann Neurol 2022).
We demonstrated that DLG4 protein is synthesized by microglia in immature mouse and human brain and developmentally regulated. Dlg4 could play a critical role in microglia reactivity as a hub protein. Genetic variation in DLG4 impacts inter-individual susceptibility to injury after preterm birth.
Using nanoparticle-based drug delivery therapy, we specially activated the WNT pathway and overexpressed the microRNA 146-5p in microglia and improving developmental trajectories of EoP mice including myelination and memory and social abilities.
Neonatal TBI induced by weight drop trauma have also demonstrated neuroinflammation processes characterized pro-regenerative and immunoregulatory microglial reactivity (Chhor V. et al., Brain Behav Immunol 2017) paving the way for an in-depth analysis of the molecular pathways driving reactivity for therapy design.
Disturbance of oligodendrocytes (OLs) maturation and myelination seem to be a common and major futures of developing brain exposed to early inflammatory events. We demonstrated that OL precursor cells and immature OLs have the capability to react to inflammation but also modulate immune reactivity of microglia. Specifically, we highlighted that immune and inflammatory pathways are developmentally downregulated along the OL maturation, and early inflammatory process in EoP mouse brain altered cell development and identity in maturing OLs (Schang AL. et al., Cell Death Dis 2022). In addition, we revealed a specific immune reactivity of OLs that play a critical role in shaping the microglia reactivity during inflammation (Boccazzi M. et al., Cell Death Dis 2021).
Contacts
Juliette Van Steenwinckel
NeuroDiderot, Inserm U1141
Hôpital Robert Debré
48 boulevard Sérurier
75019 Paris
juliette.van-steenwinckel@inserm.fr
Pierre Gressens
NeuroDiderot, Inserm U1141
Hôpital Robert Debré
48 boulevard Sérurier
75019 Paris
pierre.gressens@inserm.fr
À lire aussi
Job opportunity within The Integrative Genomics in Neurodevelopment group
The Integrative Genomics in Neurodevelopment group is seeking a highly motivated post-doctoral researcher. Check out the opportunity here: Post-doc researcher position in Computational Biology

Sleep2Develop research program
The Sleep2Develop program investigates the role of sleep as a promotor of neurodevelopment and identifies the sleep determinants of childhood development. This is rephrased as Sleep(to)Develop? or Sleep(to)Develop! Our sleep research combines clinical and...

Job opportunity
NemoClinics platform is hiring an engineer. Check out the opportunity here: U 1141 -Ingénieur-e en expérimentation et instrumentation biologiques- CDD 2023 Read more
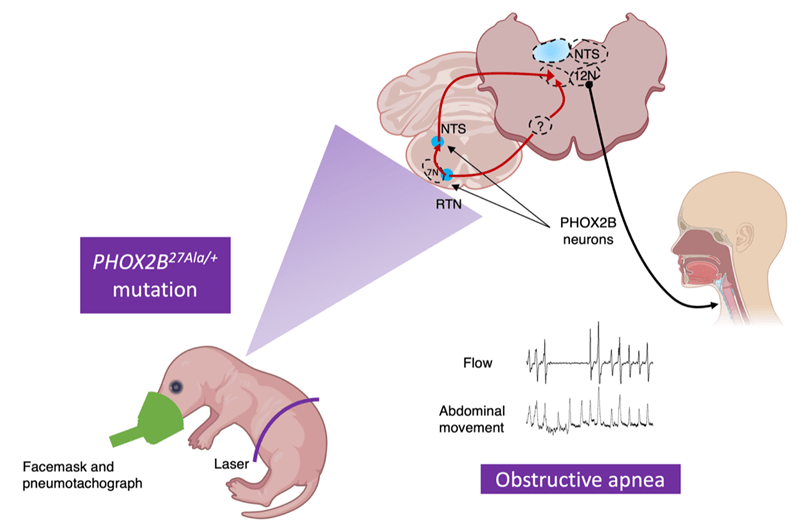
Research program on CCHS
CCHS is a disease characterized by a congenital impairment of the central control of breathing, associated with dysfunctions of the autonomic nervous system (ANS) (Gallego, 2012). At birth, patients have severe and persistent hypoventilation during sleep and a...
