Brain development disorders
Understanding mechanisms that control final neuronal output and brain size and how their alterations can lead to brain developmental disorders is a major issue in developmental neurobiology. With the aim of enhancing this understanding, we have focused our studies on the following aspects:
Neurogenesis in the human hippocampus and dentate gyrus, from embryonic stages to adulthood (Homa Adle-Biassette)
We have characterized molecular markers expressed during neurogenesis in the human hippocampus and dentate gyrus, from embryonic stages to adulthood. In addition, we have analyzed how the expression of these markers is affected in Alzheimer patients. We are now developing studies aiming a better characterization of the signaling pathways that are involved in hippocampal neurogenesis.
Brain developmental defects caused by congenital CMV infection (Natacha Teissier)
Our analyses on fetal brain infected with CMV have shown that the density of CMV-infected cells and the preferential tropism of CMV for neural stem/progenitor cells are the major factors that determine the severity of brain abnormalities, which encompass microcephaly, leukoencephalopathy, and damage to the limbic system and olfactory bulbs. We are now conducting studies to shed light on how CMV infection can alter cerebellum development.
Impairment of fueling the cell cycle during neurogenesis and MCPH (Jeannette Nardelli)
Investigation on Hereditary Primary Microcephalies (MCPH) has provided useful information on control of cortical size because a common feature of MCPH is a failure to produce adequate number of neurons during development. With this regard, we have focused our studies on the MCPH1 gene, encoding two isoforms: MCPH1-FL (full length) with three protein-binding BRCT (BRCA-CTerminal) domains and MCPH1-delC, deleted of the two C-ter BRCT domains. In the mouse developing cerebral cortex, we have shown
- that MCPH1 is expressed in radial glial cells within a short time-window preceding the bulk of neurogenesis, and its deletion causes ab-ventricular mitosis and progenitor cell death peaking at e12.5;
- the expression of MCPH1 at the mitochondria and the impact of this expression on mitochondrial activity;
- the importance of gluconeogenesis for the expansion of the early neural progenitor pool and the influence of MCPH1 on this pathway.
Overall, our data highlight a critical role of MCPH1 in adapting to the increased metabolic needs of dividing neural progenitors, leading to the idea that MCPH phenotype can result from a failure to adapt to the increased metabolic needs required to maintain proliferating progenitors in the cell cycle. Presently, we further explore this role by following these objectives:
- Determine the respective roles of the two MCPH1 isoforms, which present a divergent spatio-temporal expression pattern in human.
- Characterize further molecular mechanisms that control energetic metabolic pathways (glycolysis/gluconeogenesis versus oxidative phosphorylations) and influence the balance between proliferation/differentiation in neural progenitors, including pathways regulating MCPH1 expression.
To fulfill these objectives, we will take advantage of the following procedures and tools:
- Acute inhibition of MCPH1 expression in mouse neural progenitor using the Cre/lox system and determine how MCPH1 influences the interplay between cell cycle and energetic metabolism.
- Forced expression of the HA-tagged MCPH1 isoforms, either in vivo by in utero electroporation in the mouse (col. Colette Dehay, Inserm U1208), or in human embryonic cortical progenitors, using lentiviral inducible expression; analysis of the respective impact on the fate of neural progenitors of both species.
- Identification of molecular partners of MCPH1 at mitochondria by pull-down experiments and mass-spec analysis of the proteins co-immunoprecipated with the HA-MCPH1 isoforms transduced in Human progenitors cells (col. Marco Malumbres, CNIO, Madrid)
- Identification of MCPH1 cis-regulatory elements and transactivating pathways, and of epigenetic changes induced by MCPH1 deficiency or low glucose availability (ATAC-seq; changes in histone modifications).
Altogether, our results will help focus on whether environmental variables including nutritional status can impact genetic anomalies affecting the proliferation of neural progenitor during early stages of brain development.
Contacts
Homa Adle-Biassette
Head of the Department of Pathology
Lariboisière hospital
2 rue Ambroise Paré
7545 Paris Cedex 10
homa.adle@inserm.fr
Natacha Teissier
ORL Department
Robert Debré Hospital
48 boulevard Sérurier
75019 Paris
natacha.teissier@aphp.fr
Jeannette Nardelli
UMR Inserm U1141 – NeuroDiderot
Robert Debré Hospital
48 boulevard Sérurier
75019 Paris
jeannette.nardelli@inserm.fr
Read more
Job opportunity within The Integrative Genomics in Neurodevelopment group
The Integrative Genomics in Neurodevelopment group is seeking a highly motivated post-doctoral researcher. Check out the opportunity here: Post-doc researcher position in Computational Biology

Sleep2Develop research program
The Sleep2Develop program investigates the role of sleep as a promotor of neurodevelopment and identifies the sleep determinants of childhood development. This is rephrased as Sleep(to)Develop? or Sleep(to)Develop! Our sleep research combines clinical and...

Job opportunity
NemoClinics platform is hiring an engineer. Check out the opportunity here: U 1141 -Ingénieur-e en expérimentation et instrumentation biologiques- CDD 2023 Read more
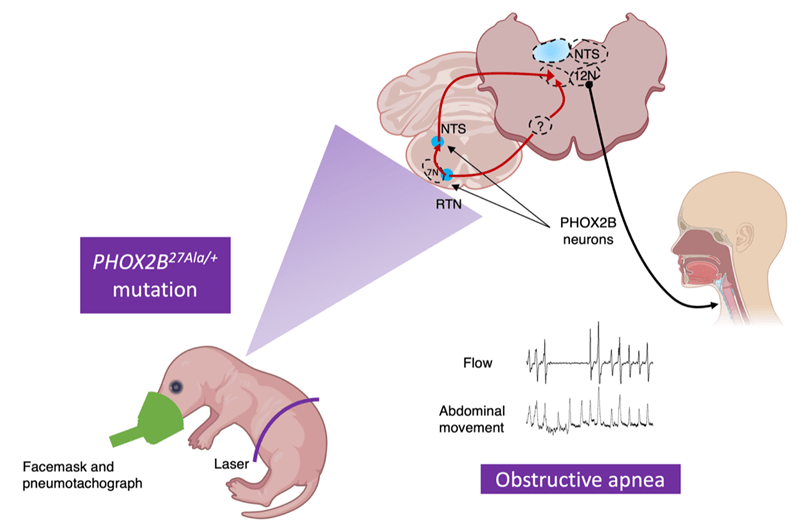
Research program on CCHS
CCHS is a disease characterized by a congenital impairment of the central control of breathing, associated with dysfunctions of the autonomic nervous system (ANS) (Gallego, 2012). At birth, patients have severe and persistent hypoventilation during sleep and a...
